Sources of radiation, a reference for radiologic technologists

Since the beginning of life on Earth, radiation has been a constant environmental factor affecting all living organisms. However, the general public is often not fully informed about the different sources of radiation that exist around us, encompassing both naturally occurring and artificially created types. It is estimated that approximately 50 percent of the radiation to which humans are exposed comes from natural sources such as cosmic rays, radon gas, and terrestrial radiation. The other 50 percent results from man-made sources, including medical procedures, industrial applications, and nuclear power production. This article delves into the wide range of radiation sources, highlighting their roles and impacts on our daily lives.
A worker manages a plutonium button. Historically, plutonium was viewed as a rare, primarily man-made element. In contemporary practices, remnants from its initial manufacturing processes, as well as plutonium reclaimed from dismantled nuclear arsenals, undergo a sophisticated reprocessing. This repurposing transforms these materials into high-quality plutonium metal, underscoring its sustained importance and utility in various applications.
Radiation surrounds us in various forms and emanates from multiple sources. It exists naturally and has been a part of the Earth’s environment since its formation. Natural radiation comes from the sun, pervades the atmosphere, and is embedded in the earth’s crust. Moreover, with the progression of technological advancements, numerous artificial or man-made sources of radiation have been developed. These include medical imaging devices, industrial equipment, and nuclear facilities. Interestingly, despite the proliferation of these man-made sources, natural sources of radiation still account for the majority of human exposure, typically four to five times that of artificial radiation.
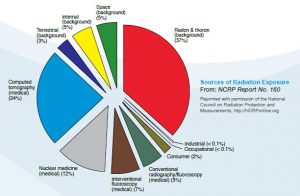
Sources of Radiation Exposure
According to information from the Nuclear Regulatory Commission (NRC), there are several common sources of routine radiation exposure. These sources encompass a wide range of natural and man-made environments. Below is a list detailing these prevalent sources:
-
- Possessing porcelain crowns or false teeth, which contribute approximately 0.07 mrem to your annual radiation exposure.
- Use gas lantern mantles when camping (0.003 mrem)
- Wear a luminous wristwatch (LCD) (0.006 mrem)
- Use luggage inspection at airports (using typical x-ray machine) (0.002 mrem)
- Watch TV (<1 mrem)
- Use a video display terminal (<1 mrem)
- Have a smoke detector (0.008 mrem)
- Wear a plutonium-powered cardiac pacemaker (100 mrem)
- Have had diagnostic x-rays (e.g., upper and lower gastrointestinal, chest) (40 mrem)**
- Have had nuclear medical procedures (e.g., thyroid scans) (14 mrem average)
- Live within 50 miles of a nuclear power plant (pressurized water reactor) (0.0009 mrem)
- Live in a stone, brick, or concrete building (7 mrem)
- Travel by jet plane (1 mrem for each 1,000 miles)
- Live within 50 miles of a coal-fired electrical utility plant (0.03 mrem)
- The NRC website has an interactive personal annual radiation dose calculator to estimate your personal dose.
Learn more about your radiation exposure
Terrestrial Radiation
Understanding Terrestrial Radiation
Terrestrial radiation is a significant component of natural background radiation, originating from radioactive materials naturally present in the earth, such as uranium, thorium, and radon. These materials contribute to the ambient radiation levels we are exposed to daily. Here are the key elements of concern in terrestrial radiation:
- Common Elements with Radioactive Isotopes: Potassium and carbon are examples of elements that, despite having radioactive isotopes in low abundance, contribute to natural background radiation.
- Long-lived Radioactive Elements: Uranium and thorium, along with their decay products such as radium and radon, are intensely radioactive despite their low concentrations. These elements have been decreasing in activity over time due to radioactive decay.
The activity of these radioactive sources has been decreasing since the Earth’s formation. For example, uranium-238’s activity today is only about half of what it was originally due to its 4.5 billion-year half-life. This gradual decline is largely imperceptible on human timescales, given the vast difference between the half-life duration of these elements and the relatively short span of human history.
The Half-life Concept
The half-life of a radioactive element is the time it takes for half of the radioactive atoms in a sample to decay. Understanding this concept is crucial to comprehending the long-term impact of terrestrial radiation:
- Uranium-238: With a half-life of 4.5 billion years, uranium-238’s activity has halved since the Earth’s formation. This slow rate of decay means its presence and radiation levels are relatively stable over the short term.
- Thorium and Radon: Similar to uranium, these elements have long half-lives and contribute to the continuous background radiation.
Minimal Impact on Human Timescales
The implications of the gradual decline in the activity of these radioactive elements are minimal over human timescales. While these elements have been decaying for billions of years, the changes in radiation levels are negligible within the relatively short span of human history.
For further information on natural background radiation and its sources, you can explore detailed resources provided by the [Environmental Protection Agency (EPA)](https://www.epa.gov/radiation/radiation-sources-and-doses).

Cosmic Radiation
Understanding Cosmic Radiation
Cosmic radiation is a significant component of natural background radiation that originates from outer space. This type of radiation consists of penetrating ionizing particles and electromagnetic waves. Here’s a detailed look at the elements and impacts of cosmic radiation:
Sources and Composition
- Cosmic Rays: These high-energy particles primarily come from beyond the Solar System. When they interact with the Earth’s atmosphere, they can create showers of secondary particles that sometimes reach the Earth’s surface.
- Solar Emissions: The sun and other stars continuously emit cosmic radiation toward Earth, similar to an unending drizzle of rain.
Factors Influencing Cosmic Radiation Intensity
The intensity of cosmic radiation is influenced by several factors:
- Elevation: The amount of cosmic radiation increases significantly at higher altitudes. For instance, passengers and crew on commercial flights experience higher levels of cosmic radiation compared to those at ground level.
- Atmospheric Conditions: The atmosphere acts as a shield, absorbing and reducing the intensity of cosmic radiation. Thicker atmospheric layers at lower altitudes provide more protection.
- Earth’s Magnetic Field: The Earth’s magnetic field deflects some cosmic radiation, reducing the amount that reaches the surface.
Impact on Human Activity
While cosmic radiation is more intense at higher altitudes, it remains relatively low in terms of overall risk. For example, approximately 100 one-way flights between Toronto and Vancouver would be required to equal the radiation exposure from other natural background sources in a single year. This comparison illustrates that, although cosmic radiation has a notable presence, its impact on everyday life is minor.
For more detailed information on cosmic radiation and its effects, visit the [Environmental Protection Agency (EPA)](https://www.epa.gov/radiation/radiation-sources-and-doses).

Impact of Cosmic Radiation and Solar Proton Events
Lithium, beryllium, and boron are not only abundant in the universe but are also often found in increased quantities within particle showers produced by cosmic rays. Additionally, cosmic rays contribute to the formation of cosmogenic isotopes, both stable and radioactive, such as carbon-14, which are generated when cosmic rays interact with the Earth’s atmosphere.
Galactic Cosmic Rays (GCRs) and HZE Ions
A significant component of galactic cosmic rays (GCRs) are the HZE ions, which are high-energy nuclei with an electric charge greater than +2. This category includes all elements heavier than hydrogen (which has a +1 charge) and helium (which has a +2 charge). HZE ions are characterized by their nuclei, which lack orbiting electrons, making their net charge equal to the atomic number of the nucleus. This property makes HZE ions particularly noteworthy in the study of cosmic radiation.
Solar Proton Events
A solar proton event, or proton storm, occurs when protons emitted by the Sun are accelerated to very high energies. These events typically happen during solar flares or due to shocks associated with coronal mass ejections. Besides protons, these phenomena can also involve other nuclear particles such as helium ions and HZE ions, leading to solar particle events.
Effects on Human Health and Safety Measures
Scientific evidence suggests that these energetic proton events do not pose significant harm to human health at ground level, particularly within the latitudinal bands where most of the Earth’s population resides. The Earth’s magnetic field plays a critical role in shielding the planet from these high-energy particles, preventing them from reaching the surface.
However, increased radiation levels have been recorded on high-altitude commercial transpolar flights during such cosmic events. To mitigate these risks, there is an established warning system that alerts pilots to these events, allowing them to reduce their cruising altitudes to safer levels, thus limiting exposure to enhanced radiation.
Impact on Space Travel
Aircraft flights that operate away from polar regions generally experience minimal impact from solar proton events. However, significant proton radiation exposure can occur for astronauts operating beyond the Earth’s magnetosphere, such as those in transit to or stationed on the Moon. The risks of proton radiation for astronauts can be significantly reduced if they remain within low Earth orbit and confine themselves to the most heavily shielded sections of their spacecraft. It’s noteworthy that proton radiation levels in low Earth orbit vary with the orbital inclination: the closer a spacecraft gets to the polar regions, the higher the exposure to energetic proton radiation.
For more detailed information on cosmic radiation and its effects, visit the [National Aeronautics and Space Administration (NASA)](https://www.nasa.gov/topics/earth/index.html) website.
Actinides
Actinides: Nature’s Radioactive Metals
Actinides are a group of naturally occurring radioactive metals, including well-known elements such as uranium and plutonium. On the periodic table, actinides encompass the 15 metallic chemical elements with atomic numbers from 89 to 103, ranging from actinium to lawrencium. All actinides are inherently radioactive, emitting energy through radioactive decay processes.
Key Actinides: Thorium and Uranium
Among the actinides, thorium and uranium are the most prevalent on Earth, having been present since the planet’s formation. These elements, along with plutonium, are critical in various applications such as nuclear reactors and nuclear weaponry. Most actinides beyond these elements are not naturally occurring but are synthesized in laboratories.
Characteristics of Actinides
Actinides share typical metallic characteristics:
- Soft and silvery in appearance
- Tarnish when exposed to air
- Dense and malleable
- Some are so soft they can be sliced with a knife
Within the actinide series, there are two overlapping groups:
- Transuranium Elements: Elements beyond uranium in the periodic table
- Transplutonium Elements: Elements beyond plutonium
These subgroups share similar properties and enhance our understanding of chemical and nuclear science.
Practical Uses of Actinides
Actinides are not only fundamental to advanced scientific applications but also have practical uses in everyday life. For example:
- Smoke detectors utilize small amounts of americium-241.
- Gas mantles often contain thorium to improve brightness.
Beyond these everyday applications, actinides are crucial as fuel in nuclear reactors and as key components in nuclear weapons, highlighting their importance in both energy production and national defense strategies.
For more detailed information on actinides and their applications, visit the [Los Alamos National Laboratory](https://www.lanl.gov) website.

The Periodic Table highlighting Actinides in the bottom row, including thorium and uranium, the two most prevalent radioactive elements on Earth.
The Role of Uranium-235 in Nuclear Power
In the realm of nuclear power, uranium-235 is a crucial isotope due to its use in thermal reactors, the most common type of nuclear reactor. Additionally, other actinide isotopes such as thorium-232 and uranium-233 hold promise for future applications in nuclear energy, thanks to their potential utility in different reactor designs.
Plutonium-238: A Versatile Radioactive Isotope
Plutonium-238 is a significant isotope within the actinide series, boasting a half-life of 87.7 years. Its primary utility stems from being a potent alpha emitter, which releases minimal amounts of more penetrating and hazardous types of radiation. These characteristics make plutonium-238 extensively useful in radioisotope thermoelectric generators (RTGs) and radioisotope heater units (RHUs). These devices are critical for supplying heat and electricity in spacecraft and other remote applications where traditional power sources are unfeasible.
Applications of Plutonium in Nuclear Technology
Plutonium, a key member of the actinide series, is predominantly used in nuclear weapons and as fuel in nuclear reactors due to its high fissionable properties. Beyond terrestrial applications, Plutonium-238 has found utility in space exploration, powering thermopiles and water distillation systems aboard some satellites and space stations. This isotope’s ability to release steady amounts of heat over extended periods without the need for recharging makes it particularly valuable in such challenging environments.
Plutonium-238 in Medical Technology
Plutonium-238 is also notable for its use in medical technology. It serves as a long-lasting energy source in cardiac pacemakers. This application is especially beneficial because Plutonium-238 can power these devices for durations approximately five times longer than conventional batteries. This significantly enhances the quality of life for patients by reducing the frequency of surgical battery replacements.
For more information on the applications and safety of plutonium-238, visit the [U.S. Department of Energy](https://www.energy.gov/) website.

A pellet of Plutonium-238, destined for use in a thermoelectric generator on a space mission, emits a visible glow due to its own thermal energy. This pellet, capable of producing 62 watts of heat, glows because of the intense heat generated through its radioactive decay, primarily via alpha particle emission.
The Rocky Flats Fire Incident
On September 11, 1957, a significant incident occurred at the Rocky Flats Plant, a nuclear weapons production facility near Denver, Colorado. A fire broke out in one of the gloveboxes used to handle radioactive materials, specifically metallic plutonium. Plutonium is known for its pyrophoric properties and potential to ignite spontaneously under certain conditions.
Causes of the Fire
The fire began when the combustible rubber gloves and plexiglass windows within the glovebox caught fire, possibly triggered by plutonium’s reaction with air at room temperature. Plutonium’s highly reactive nature made it a catalyst for the fire under the right conditions.
Consequences of the Incident
- Contamination of Building 771: The fire caused extensive contamination throughout Building 771, significantly impacting the facility’s safety and operational integrity.
- Release of Plutonium Particles: The incident resulted in the release of plutonium particles into the atmosphere, posing substantial health and environmental risks.
- Health Risks: Inhalation of plutonium particles can lead to severe health issues, including lung cancer and other radiological illnesses.
- Environmental Impact: The dispersion of plutonium into the surrounding environment raised serious concerns about long-term ecological damage.
For further reading on the Rocky Flats incident and its implications, visit the [U.S. Environmental Protection Agency (EPA)](https://www.epa.gov/) website.
Radon
Radon: The Silent Threat
Radon is the primary source of public exposure to naturally occurring ionizing radiation and often represents the most significant portion of an individual’s background radiation dose. The concentration of radon can vary greatly depending on geographic location. This radioactive gas originates from natural sources and can accumulate in buildings, particularly in enclosed areas such as basements and attics, as well as in some spring waters and hot springs.
Health Risks of Radon
There is substantial epidemiological evidence linking high concentrations of radon with an increased risk of lung cancer. Consequently, radon is recognized globally as a major indoor air quality contaminant. The United States Environmental Protection Agency (EPA) ranks radon as the second leading cause of lung cancer in the U.S., following cigarette smoking.
Radon’s Chemical Properties
Radon is a chemical element designated by the symbol Rn and atomic number 86. It is a radioactive noble gas that is colorless, odorless, and tasteless, forming naturally as a secondary decay product of uranium or thorium. As one of the densest gases under normal conditions, radon remains gaseous, making it difficult to detect without specialized equipment.
Challenges in Studying Radon
The radioactivity of radon presents significant health hazards, contributing to its classification as a major indoor air pollutant. Due to its high radioactivity, conducting chemical studies on radon is challenging, resulting in only a few known compounds of the element.
For more information on radon and its health effects, visit the [U.S. Environmental Protection Agency (EPA) Radon Page](https://www.epa.gov/radon)
Illustration of a Radon Atom. Known for being one of the densest gases under standard conditions, radon significantly contributes to environmental background radiation levels.
Radon in Homes
The issue of high radon levels in homes gained public attention unexpectedly in 1984. This came to light when an engineer working at a nuclear power plant was found to be contaminated with radioactive substances that were traced back to his own home, not the plant. This incident revealed that radon gas from soil is a primary source of indoor radon problems. Radon can infiltrate homes through various pathways, including the ground, well water, and, less commonly, certain building materials that emit radon. This awareness has heightened the focus on radon mitigation and safety measures in residential settings.
Sources of Radon in Homes
Radon primarily enters homes through:
- The ground: Radon moves up from the soil and into homes through cracks in floors and walls, construction joints, and gaps around service pipes.
- Well water: Radon can be released into the air in homes through the use of well water.
- Building materials: Certain materials can emit radon, but they rarely contribute significantly to overall radon levels.
Testing for Radon
To identify and address potential radon problems, testing is strongly recommended by the Environmental Protection Agency (EPA). There are various testing methods, with short-term options ranging from a few days to several months, allowing homeowners to quickly assess radon levels and take necessary actions if elevated levels are detected.
Short-Term Testing
The most prevalent radon testing devices include:
- Charcoal canisters
- Electret ion detectors
- Alpha track detectors
For accurate short-term testing, conduct tests in the lowest living area of the home, such as a basement, where radon levels are typically highest. Keep all doors and windows closed during the test, which is best performed during cooler months when homes are less ventilated, allowing for more accurate radon accumulation assessment.
Long-Term Testing
Long-term testing, extending up to a full year, provides the most accurate assessment of average radon concentration over time. Common devices for long-term testing include alpha track detectors and electret ion detectors. When selecting a test kit, ensure it is from a “qualified” company. Information on identifying a “qualified” radon service professional is available on the EPA’s website at www.epa.gov/radon/radontest.html. Additionally, many state radon offices provide lists of radon measurement companies that comply with state-specific requirements.
The Importance of Testing
Nearly one out of every 15 homes in the U.S. is estimated to have elevated radon levels, underscoring the importance of regular and accurate radon testing. By being proactive about radon testing, homeowners can ensure their living environments are safe and mitigate potential health risks associated with prolonged radon exposure.
The EPA outlines specific steps for testing radon levels effectively in your home:
- Step 1: Conduct a short-term radon test. If the result indicates 4 pCi/L or higher, it is recommended to proceed with a follow-up test to confirm the findings.
- Step 2: Follow up with either a long-term test for a more comprehensive analysis or a second short-term test to verify the initial results.
For more detailed information on radon testing, visit epa.gov.
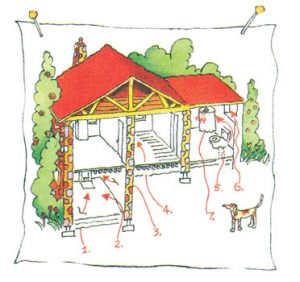
Diagram illustrating potential radon entry points in a typical home.
RADON GETS IN THROUGH:
-
- Cracks in solid floors
- Construction joints
- Cracks in walls
- Gaps in suspended floors
- Gaps around service pipes
- Cavities inside walls
- The water supply.
Naturally Occurring Food Radiation

Bananas are known to contain radioactive isotopes of potassium.
It’s a lesser-known fact that nearly all foods naturally contain small amounts of radioactivity, even without exposure to man-made sources. Among these, bananas are notably radioactive due to the presence of the radioactive isotope potassium-40 (40K). Collectively, all food sources contribute to approximately 40 millirems of radiation per person each year, accounting for over 10% of the average total radiation dose from both natural and man-made sources combined. To put this into perspective, there is a measurement known as the banana equivalent dose, which estimates the amount of radiation exposure from consuming just one banana.
Understanding the Banana Equivalent Dose
Radiation leaks from nuclear plants are often quantified in extremely small units, such as the picocurie, which is a trillionth of a curie. To make these measurements more understandable, comparisons are sometimes made to the banana equivalent dose—an informal measurement equating radiation exposure to the amount received by eating a single banana. For example, the cumulative dose from eating one banana each day over the course of a year (365 bananas in total) amounts to about 3.6 millirems (or 36 microsieverts). This comparison helps provide a clearer, more relatable understanding of the actual risks associated with radiation exposure from nuclear plant incidents.
Other Potassium-Rich, Radioactive Foods
In addition to bananas, other potassium-rich foods also contain the radioactive isotope potassium-40 (40K). These include:
- Potatoes
- Kidney beans
- Sunflower seeds
- Various nuts
Notably, Brazil nuts contain not only high levels of 40K but also radium, with radioactivity levels reaching up to 444 Bq/kg (12 nCi/kg) — nearly five times that of bananas.
Health Implications and Homeostasis
There is no need to eliminate these potassium-rich foods from your diet. Health experts, including those at the EPA, explain that the levels of potassium—and by extension, 40K—in the human body are regulated by homeostasis. This means any excess potassium absorbed from food is promptly balanced by the body’s natural elimination processes. Therefore, the consumption of these potassium-rich foods does not pose a significant health risk.
For further details on radiation in food and its health impacts, you can visit the Environmental Protection Agency’s radiation protection page.
Nuclear Fallout
Nuclear fallout, often referred to simply as fallout or historically as “Black Rain,” is the residual radioactive material propelled into the upper atmosphere after a nuclear explosion or a nuclear reaction that occurs in an unshielded facility. The term “fallout” comes from how this material “falls out” of the sky once the explosion and the subsequent shock wave have dissipated. This fallout typically includes radioactive dust and ash produced when a nuclear weapon explodes, though similar materials can also be released from a compromised nuclear power plant.
This radioactive dust consists of materials that were either directly vaporized by the nuclear explosion or those that were irradiated by the exposure. It represents a highly dangerous form of radioactive contamination that poses significant health risks.

Per capita thyroid doses in the continental United States from Iodine-131 resulting from all routes of exposure from all atmospheric nuclear tests conducted at the Nevada Test Site.
Understanding Fallout Distribution
Following an air burst during a nuclear explosion, several phenomena occur. Fission products, un-fissioned nuclear material, and residues from the weapon that were vaporized by the intense heat of the fireball quickly condense into a fine suspension of tiny particles, ranging from 10 nanometers to 20 micrometers in diameter. These particles can be rapidly carried into the stratosphere, especially if the yield of the explosion exceeds 10 kilotons. Once in the stratosphere, the particles spread globally and pose a persistent environmental hazard.
Long-term Radiobiological Risks
The radiobiological risk associated with worldwide fallout is primarily a long-term concern. This risk stems from the potential for long-lived radioisotopes, such as strontium-90 and caesium-137, to accumulate in the human body through the consumption of contaminated food. The health implications of these isotopes are profound as they can integrate into bone and muscle tissue, leading to various radiation-induced conditions. While the global fallout presents a significant long-term threat, it is the local fallout—material that falls to the ground within a few hours of an explosion—that often requires more immediate attention due to its higher initial radiation levels and direct impact on the surrounding area.
Surface Burst Fallout
In the event of a nuclear explosion that occurs at or near the surface of the land or water, the intense heat generated by the blast vaporizes significant amounts of earth or water. This vaporized material is then drawn up into the resulting radioactive cloud. As it ascends, it mixes and condenses with fission products and other radioactive contaminants that have been activated by neutrons. This process imbues the vaporized earth or water with radioactivity.
When the radioactive cloud eventually settles back to the ground, it contaminates large expanses of land and bodies of water. This widespread contamination introduces radiation into ecosystems and drinking water supplies, which can lead to profound and lasting biological effects. The resultant radiation exposure can cause genetic mutations and other severe health impacts across a wide range of animal and human populations, thereby altering the affected ecosystems for generations.
Immediate Fallout Hazards
A nuclear explosion at the surface generates a significant amount of particulate matter, ranging from ultrafine particles less than 100 nanometers in diameter to larger fragments several millimeters across. This mix includes extremely fine particles that contribute to global fallout. In the dynamics of a surface burst, the larger particles typically spill out from the stem of the mushroom cloud and cascade downward in a downdraft even as the main cloud continues to rise. This process causes fallout to begin settling near the blast site within an hour of the explosion.
Local fallout, which consists of over half of the total debris from the bomb, tends to land on the ground within approximately 24 hours of the explosion. The deposition rates of these particles are influenced by the chemical properties of the elements involved; less volatile elements tend to settle to the ground first. This immediate deposition of radioactive material poses a severe hazard to the environment and public health in the vicinity of the explosion site.
Weather Influence on Fallout
Severe local fallout contamination from a high-yield surface detonation can extend far beyond the immediate blast area, affecting regions well away from the explosion site. The spread and severity of fallout are heavily influenced by weather conditions at the time of the detonation and thereafter.
Key Weather Influences:
- Wind: Strong winds can carry fallout particles over greater distances, expanding the affected area. However, while high winds spread fallout more widely, they also dilute it, reducing concentration levels as the particles disperse.
- Fallout Pattern: The width of the fallout pattern at any given radiation dose rate tends to be narrower in areas with higher wind speeds, as the fallout travels further downwind. Despite these variations in spread, the total radioactive material deposited by a certain time remains constant, regardless of wind patterns.
- Thunderstorms: Weather conditions like thunderstorms can dramatically alter fallout deposition. Thunderstorms can cause radioactive materials to precipitate more rapidly through “washout” (where the mushroom cloud is beneath the thunderstorm) and “rainout” (where the mushroom cloud mixes with the storm). This can result in localized areas with significantly higher radioactivity.
Impact of Weather on Fallout Distribution:
- Wind Effects: While strong winds increase the distance fallout travels, they also cause the radioactive particles to be more spread out and less concentrated. This can affect the severity of contamination in different regions, influencing cleanup efforts and long-term environmental impacts.
- Rainfall Effects: Rain can cause fallout to deposit more quickly and intensely. Thunderstorms, in particular, can lead to higher concentrations of radioactive material in localized areas, complicating remediation efforts and posing greater immediate health risks.
Overall Implications:
Understanding how weather conditions affect fallout distribution is crucial for planning effective response and mitigation strategies. The role of wind and rain in dispersing and depositing radioactive materials must be considered to address both immediate and long-term environmental and health consequences.
For more detailed information on fallout and its effects, you can visit the [Centers for Disease Control and Prevention’s Radiation Emergencies page](https://www.cdc.gov/nceh/radiation/emergencies/index.htm).
Exposure and Health Risks
Remaining in areas contaminated by radioactive materials leads to direct external radiation exposure and potential internal hazards due to the inhalation and ingestion of radioactive contaminants. One notable radioisotope is iodine-131, which, despite its relatively short half-life, poses significant health risks because it tends to accumulate in the thyroid gland.
Key Health Risks of Iodine-131:
- Thyroid Accumulation: Iodine-131 is particularly dangerous as it accumulates in the thyroid gland, increasing the risk of thyroid cancer and other thyroid-related diseases.
- Short Half-Life, High Impact: Despite its short half-life of about 8 days, iodine-131 can cause considerable health issues due to its concentrated impact on the thyroid.
Historical Context and Safety Measures:
- Nuclear Testing Impact: According to a 1992 report from the National Cancer Institute, about 150 million curies of radioactive iodine were released into the atmosphere during nuclear testing in Nevada from the early 1950s to the early 1960s. This extensive release contaminated much of the nation’s milk supply.
- Contamination Pathway: Fallout particles, including iodine-131, settled on grazing fields where dairy cows fed. The contaminated grass led to the transfer of radioactive material into the milk produced by these cows, posing a significant health risk to consumers.
Modern Safety Protocols:
To mitigate such risks, modern safety protocols involve regular collection and testing of pasteurized milk samples at dairy plants for signs of radioactivity. This ensures the safety and health of the public by preventing contaminated milk from reaching consumers.
For more detailed information on radiation exposure and health risks, visit the [National Cancer Institute’s Radiation Exposure page](https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation).
Internal exposure to radioactive iodine-131 through ingestion.
Human Bodies
Several essential elements that constitute the human body, particularly potassium and carbon, contain radioactive isotopes that contribute to our natural background radiation exposure. As a result, all individuals inherently possess internal radiation, primarily from radioactive potassium-40 and carbon-14, which have been present within their bodies since before birth. Consequently, every person inherently emits a small amount of radiation, thereby exposing those around them. However, the variation in radiation dose from one person to another due to these isotopes is relatively minor compared to the fluctuations associated with cosmic and terrestrial radiation sources.
Vitruvian Man by Leonardo Da Vinci. Even all human bodies normally contain some traces of radioactive isotopes.
Depleted Uranium
Depleted uranium (DU) is a form of uranium with a reduced concentration of the fissile isotope U-235 compared to natural uranium. This material is notable for its extremely high density, measuring 19.1 g/cm3, which is 68.4% denser than lead. This unique property makes depleted uranium valuable for a variety of applications.
Civilian Uses:
- Aircraft Counterweights: Due to its weight, DU is commonly used as counterweights in aircraft, providing necessary balance.
- Radiation Shielding: DU is employed in radiation shielding for medical radiation therapy and industrial radiography equipment, effectively blocking harmful radiation.
- Transportation Containers: It is used in containers designed for the safe transportation of radioactive materials, leveraging its protective qualities against radiation.
Military Applications:
- Armor Plating: DU is used in defensive armor plating due to its high density and strength.
- Armor-Piercing Projectiles: DU is also used in armor-piercing projectiles. The incorporation of depleted uranium in military munitions has sparked significant controversy due to concerns over potential long-term health effects associated with its use.
Health Concerns:
- Toxicity: Uranium is a toxic metal, and exposure can adversely impact the functioning of vital organs and systems, including the kidneys, brain, liver, and heart.
- Chemical Hazard: The primary health threat from depleted uranium is its chemical toxicity rather than its radiological aspects.
Radiation Aspects:
- Weak Radioactivity: DU exhibits weak radioactivity due to the long half-lives of its isotopes—uranium-238 (about 4.468 billion years) and uranium-235 (about 700 million years).
- Biological Half-Life: The average time for the human body to eliminate half of the ingested or inhaled uranium is about 15 days, meaning it tends to be cleared from the body relatively quickly.
- Alpha Particles: The primary radiation hazard comes from the emission of alpha particles, which have very limited penetration abilities and do not travel far through air or penetrate clothing. Therefore, the main risk is internal exposure through inhalation, ingestion, or contamination via shrapnel wounds.
Safety Precautions:
- Internal Exposure: The main concern with depleted uranium is internal exposure. While there are radiation risks, the chemical toxicity poses a greater health threat, requiring careful handling and precautions to avoid internal exposure.
For further details on depleted uranium and its health impacts, you can visit the [Environmental Protection Agency’s radiation protection page](https://www.epa.gov/radiation/radiation-sources-and-doses).

Gunner’s mates inspect linked belts of Mark 149 Mod 2 20mm ammunition before loading it into the magazine of a Mark 16 Phalanx close-in weapons system aboard the battleship USS MISSOURI.
Other Man-Made Sources
While medical procedures are recognized as the primary man-made source of radiation exposure for the public, various consumer products and materials also contain trace amounts of radioactive substances.
Construction Materials
- Building homes and roads
- Combustible fuels like gas and coal that release radiation during combustion
Everyday Devices
- X-ray security systems
- Televisions
- Fluorescent lamp starters
Household Items
- Smoke detectors (utilizing americium)
- Luminous watches
- Tobacco smoke
Ceramics
Certain ceramics also contain radioactive materials, adding to the diverse array of man-made sources that contribute to environmental radiation.
External Resources:
- [Nuclear Regulatory Commission – Consumer Products](https://www.nrc.gov/materials.html
- [Environmental Protection Agency – Radiation Sources](https://www.epa.gov/radiation/radiation-sources-and-doses)
Internal References:
– [Company Policy on Radiation Safety]
– [Safety Procedures for Handling Radioactive Materials]

“Smoking Club” – An illustration included in Frederick William Fairholt’s Tobacco, its history and associations. Tobacco smoke is known to contain radioactive elements.
The Role and Risks of Cobalt-60 in Industrial Applications
Cobalt is a significant component in steel alloys, enhancing their properties for various applications. A notable radioactive isotope of cobalt, Cobalt-60 (60Co), is extensively used in medical and industrial settings, particularly for the sterilization of equipment and supplies. However, the management of Cobalt-60, especially its disposal, poses challenges.
Issues with Cobalt-60 Disposal
Improper disposal of 60Co in scrap metal has led to unintentional radioactivity in numerous iron-based products. This contamination occurs when scrap metal containing residual 60Co is recycled and reused in the production of new iron and steel products, underscoring the importance of rigorous controls and monitoring in the handling of radioactive materials.
Notable Incidents of Contamination
Petco Recall in 2012
In August 2012, Petco issued a recall for several models of steel pet food bowls following an alert from US Customs and Border Protection, which discovered that the bowls were emitting low levels of radiation. Further investigations revealed that the source of this radiation was Cobalt-60 (60Co), a radioactive isotope that had inadvertently contaminated the steel used to manufacture the bowls.
This incident highlights the critical need for careful monitoring and regulation of recycled materials in the manufacturing process to prevent radioactive contamination in consumer products. More information on this recall can be found on Petco’s official website: Petco Recall Information.
Bed, Bath & Beyond Tissue Boxes in 2012
In January 2012, an alarming discovery was made when Metal Boutique tissue boxes manufactured in India and distributed to Bed, Bath & Beyond stores across 20 states were found to contain radioactive Cobalt-60 (60Co). This incident highlighted the risks associated with inadequate monitoring of imported goods.
More information about the tissue box recall can be found here: Bed, Bath & Beyond Recall Information.
Asos Metal-Studded Belts in 2013
A similar case occurred in May 2013 when a batch of metal-studded belts sold by the online retailer Asos tested positive for 60Co and had to be confiscated. These belts were subsequently held in a U.S. radioactive storage facility to prevent public exposure. Such events underscore the importance of rigorous testing and regulation of imported products to ensure they meet safety standards.
“Such events underscore the importance of rigorous testing and regulation of imported products to ensure they meet safety standards.”
These incidents highlight the critical need for stringent controls and monitoring of Cobalt-60, particularly in recycled materials, to prevent radioactive contamination in consumer products.
To learn more about orphaned sources of radiation, visit: Read more about orphaned sources of radiation.
For further details on radiologic technology and continuing education, explore our resources at CE4RT.
Radiation From Medical Procedures
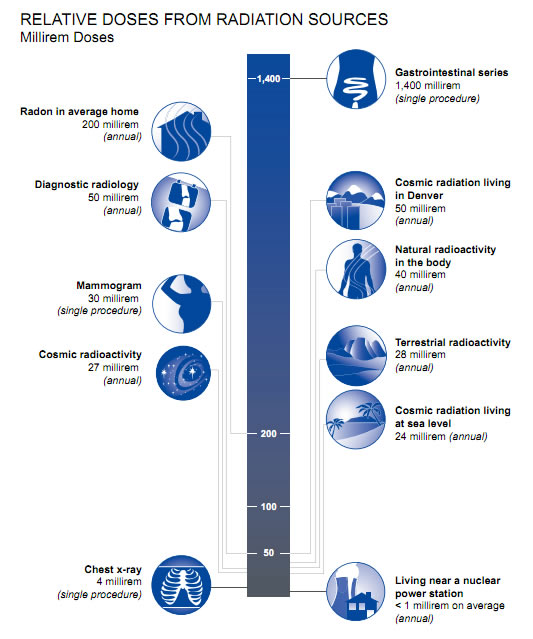
The overwhelming majority of man-made radiation exposure encountered by the general population originates from medical procedures. The table below compares various types of man-made radiation exposure against radiation-based medical procedures, organizing them by the level of exposure measured in Sieverts (Sv).
Comparing Radiation Exposure
Additionally, the table includes a column that approximates how long it would take for an individual to receive the equivalent dose of radiation from natural environmental sources. This comparison aims to offer some perspective on the radiation doses associated with medical imaging and treatments, particularly for patients concerned about the risks of radiation.
| Procedure | Exposure (Sv) | Equivalent Natural Exposure |
|---|---|---|
| Chest X-ray | 0.1 mSv | ~10 days |
| Mammogram | 0.4 mSv | ~7 weeks |
| CT Abdomen/Pelvis | 10 mSv | ~3 years |
| Radiation Therapy | 20-80 Sv | Up to thousands of years |
Factors Affecting Radiation Dose
It’s important to note that actual radiation doses can vary widely due to:
- Medical facility protocols
- Specific equipment used
- Patient’s size
- Other clinical considerations
“The radiation dose values listed are based on typical effective doses for each type of scan.”
However, these doses are not absolute and can differ significantly based on various factors.
Minimizing Radiation Exposure
Subsequent chapters will discuss strategies to minimize radiation exposure. As healthcare providers, there is a fundamental responsibility to ensure that radiation levels are kept as low as reasonably achievable (ALARA) to protect patient health.
For further details on occupational radiation exposure limits, visit CE4RT’s Occupational Radiation Exposure Limits.
Understanding and managing radiation exposure is crucial for both healthcare providers and patients. By staying informed and adhering to best practices, we can effectively minimize the risks associated with medical radiation.
For more information on radiography and continuing education credits, explore our comprehensive resources at CE4RT.
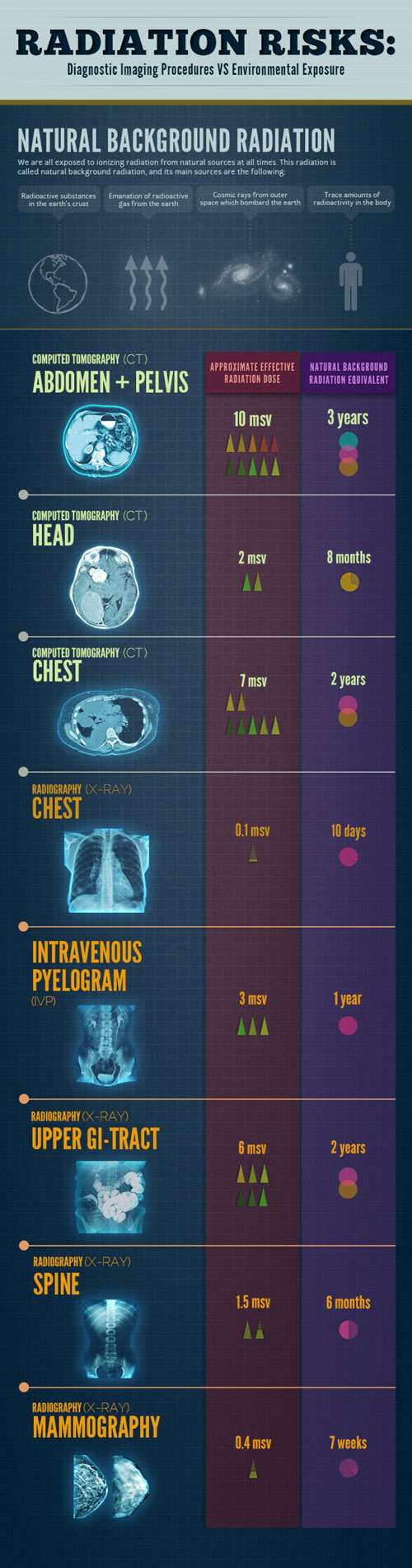
| Type of Radiation Exposure | Approximate Dose in mSv |
Approximate Equivalent Time Period of Natural Background Radiation |
| Airport Security X-ray scanner | 0.0001 | Less then 1 hour |
| 7 hour airplane flight | 0.003 | A few days |
| Chest X-ray | 0.1 | 1 week |
| Mammogram | 0.4 | 2 months |
| Chest CT | 7 | 2.3 years |
| Flouroscopy BE | 8 | 2.7 years |
| CT Heart Angiography | 16 | 5.3 years |
| Whole body PET scan | 14 | 4.6 years |
| Flouroscopy KUB | 15 | 5 years |
| CT Whole body | 22.5 | 7.5 years |
| Nuclear Medicine Cardiac Stress Thalium |
40.7 | 13.6 years |
| Transjugular Intrahepatic Portosystemic Shunt Placement |
70 | 23.3 years |
Sources of radiation exposure along with typical doses and compared to the time it would take to receive a similar dose from natural background radiation. (adapted from Mettler, Jr. FA, Huda W, Yoshizumi TT, and Mahesh M. (July 2008) Effective Doses in Radiology and Diagnostic Nuclear Medicine: A Catalog, Radiology, 248(1): 254-26)
Here is more details about arrt® ce credits online.
FAQs
1. What are the primary sources of natural background radiation?
Natural background radiation primarily comes from three sources: cosmic radiation from space, terrestrial radiation from radioactive materials in the earth, and internal radiation from radioactive isotopes naturally present in the human body. These sources contribute to the constant low-level radiation exposure that all living organisms experience.
2. How does cosmic radiation contribute to our overall radiation exposure?
Cosmic radiation originates from outer space and the sun, and it contributes to our overall radiation exposure by penetrating the Earth’s atmosphere. The intensity of cosmic radiation increases with altitude and latitude, meaning people who live at higher altitudes or fly frequently are exposed to higher levels of cosmic radiation.
3. What are some common sources of man-made radiation?
Common sources of man-made radiation include medical imaging procedures (such as X-rays, CT scans, and nuclear medicine), radiation therapy for cancer treatment, industrial applications (such as radiography and nuclear power plants), and consumer products (such as smoke detectors and certain types of luminous watches). These sources contribute to additional radiation exposure beyond natural background levels.
4. How do medical imaging procedures contribute to radiation exposure?
Medical imaging procedures contribute to radiation exposure through the use of X-rays, CT scans, and nuclear medicine. These procedures involve the use of ionizing radiation to create images of the body’s internal structures, which helps in diagnosis and treatment planning. While beneficial, it’s important to minimize exposure by using the lowest effective dose and adhering to safety protocols.
5. What measures can be taken to minimize radiation exposure from natural and man-made sources?
To minimize radiation exposure, several measures can be taken:
- Following the ALARA (As Low As Reasonably Achievable) principle in medical imaging and radiation therapy.
- Using protective shielding and personal protective equipment (PPE) when working with radiation sources.
- Limiting time spent near radiation sources and increasing distance from them.
- Regularly maintaining and calibrating radiation equipment to ensure it operates correctly and safely.
- Staying informed about radiation safety practices and adhering to regulatory guidelines and recommendations.
These measures help reduce unnecessary exposure and protect both patients and professionals from the potential harmful effects of radiation.
